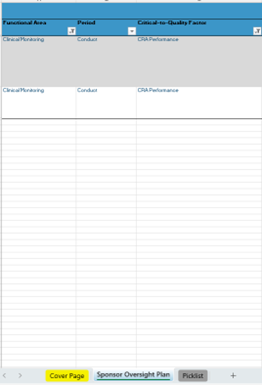
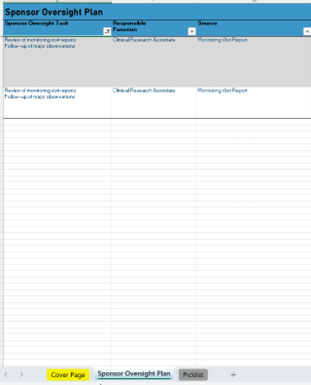
Sponsor Oversight Plan
-
User Group
Professionals Involved in Clinical Trials
-
Format
Microsoft Excel
-
Content
- Classify per Functional Area and Phase
- Define your “Critical-to-Quality” Factor
- Define your Sponsor Oversight Activity and Responsibility
- Define your Quality Tolerance Limits
- Document your Sponsor Oversight Activities
-
Benefits
- Structured Documentation for your Sponsor Oversight Plan
- Structured Documentation for your Sponsor Oversight Activities