Risk-based
TMF Quality Control
The Trial
Master File (TMF) is the collection of essential documents that demonstrate
the Sponsor's compliance with the applicable regulations and guidelines, as
well as the quality and validity of the clinical trial data. The TMF also
serves as a source of information for audits and inspections by regulatory
authorities or other authorized parties.
A risk-based
approach to TMF Management allows the Sponsor to prioritize the most
critical and relevant aspects of the TMF and allocate resources accordingly.
A
risk-based approach also helps the sponsor to adapt to changing circumstances
and address emerging issues in a timely and proportionate manner.
Regular
quality control (QC) of the TMF is an essential part of the Sponsor Oversight
process. QC activities ensure that the TMF is complete, accurate, consistent,
and up-to-date, and that any gaps, errors, or discrepancies are detected and
resolved promptly.
QC also
verifies that the TMF reflects the current status and progress of the clinical
trial and complies with the protocol, the functional plans, and the applicable
standards and regulations.
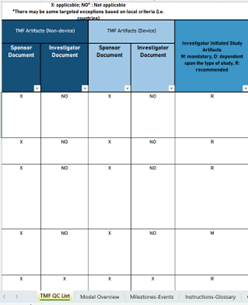
This Template already includes many examples of the
criticality assessment and auto-calculated risk scores. Thus, you have a good starting
point for your risk-based TMF QC.
-
User Group
Professionals
Involved in TMF Quality Control
-
-
Content
- Based on the
DIA Reference Model v3.3
- Define the
Criticality per Document
- Define the
Document Status
- Accept or
Deny the Risk per Document
- Define
your Quality Tolerance Limits
- Define
your Risk Mitigation
-
Benefits
- Documentation for your risk-based TMF Quality Control Plan
- Documentation for your risk-based TMF Quality Control Process
With a risk-based
TMF Quality Control, you will
- Ensure
the Completeness, Accuracy and Timeliness of your TMF documentation throughout
the Clinical Trial Lifecycle
- Identify and address potential Issues or Gaps in your TMF before they become
critical or affect your Inspection Readiness
- Reduce
the Cost and Effort of conducting full TMF Reviews by implementing ongoing Quality
Checks focused on Critical Documents
- Align
your TMF Quality Control activities with the ICH-GCP E6(R3) guidelines and the
risk-based Quality Management Principles
- Enhance
the Transparency and Accountability of your TMF Stakeholders and improve Collaboration
across Sites, Service Providers and Sponsor